#1 Explanation
Explanation of some of the
units:
Northing and Easting are UTM coordinates from
the GPS.
Conductivity is in
microsiemens per centimeter. Don’t know what
that means, but the higher the conductivity, the
higher the concentration of dissolved ions
(salts and the like).
TDS is total dissolved
solids, and should include organic and inorganic
solids.
Salinity in parts per
thousand (same as grams per liter). My
recollection is that it’s a measure of the level
of chloride ions in the water.
"Half-informed comments on the
results." quoted
Conductivity and TDS are (not
surprisingly) much higher than in the lakes we have
been covering for MTRI. Conductivities in those lanes
ranged from 25.4 (Kegeshook, .25 m depth) to 45 (.25 m
depth, Sloans) and TDS ranged from 17.55 (lower depths
of Kegeshook) to 33.15 (Sloans at 18 m.).
Oxygen: Were very high in the
top meter. At 2 m., they were a little on the low
side., but not strikingly so, considering that this is
a small lake and the winds were very slight when we
hit. The supersaturation in the top meter, however, is
very interesting. It was consistent. Yes, maybe that
brown water is due to algal cells; it sort of “looked”
like phytoplankton when I looked at it, but that is
not good enough to render a diagnosis. Another thing
that occurs to me is that the upper water probably
warmed up pretty fast this morning. Warming would
drive some of the dissolved gases out, but there would
be a lag period.
It would be interesting to see
what the surface oxygen look like on a very overcast
day or at first light, when oxygen levels should be at
a minimum.
pH: Wow! Again, the level at 2 m
off Arnold’s wharf (6.92) is more what you would
suspect. I am not sure what is driving up the pH near
the surface, and am also not sure what effect that is
having on whatever is making the lake brown. As I
said, I will bug Mike and see if he has any thoughts.
By the way, I did take your suggestion and check the
pH on the shore of Hectanooga Lake near the fire
station (nice, clear water!). It was 6.96-6.97, oh,
yeah, and it was also supersaturated with oxygen, but
I was getting punchy, so could not remember to what
extent.
#2. Explanation
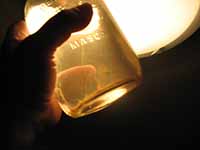
I took a look at the results. Based on the results, and
my interpretations, I don't see anything alarming. I see
that it's a shallow lake (a maximum depth of 2.7 meters
[I also hear that there is a minimum discharge into
other river/lake systems from this lake?]). The Secchi
Depth is very shallow meaning that the lake is very
turbid (this in itself is an aesthetic objective and
should only be interpreted as such (turbidity can be
caused my numerous sources) however it is also used to
correlate the amount of algae in a system ... this
correlation does have it's flaws). I'm not concerned
with the conductivity, TDS, or salinity because I
believe they are low (and I can't find any guidelines on
the parameters except for drinking water quality), the
DO results look fine (maybe slightly above average), but
I am more concerned with the pH, although once again, it
is an aesthetic objective and can be caused by numerous
sources. Please keep in mind that this is my opinion
based on interpreting the results. I know we had lots of
rain this summer, and I would assume that the most
likely suspect would be run-off from the local
environment [i.e.: organic matter such as tannin and
maybe some in/organic iron compounds]. Also, because of
the low discharge rate of the lake, it might not had
enough time to rid itself of the run-off. The only thing
about tannin, I would except it would produce an acid
environment (pH<7) rather than a basic one (pH>7).
If there were nutrient overloading, I would except the
conductivity, TDS, and DO values to be higher. However,
with more data, done over time, so that a reliable
baseline can be established, a more detailed cause could
be made. I hoped this helped.
#3
I would
guess that:
- you have excessive O2 in the surface layers
because of daytime algal production
- I'll have to check on the effects that might
have on PH
- At night, much of that O2 will be consumed by
those same organisms: makes an interesting daily
pattern
- Your thermocline is somewhere around 1.5-2 meter
and below that you see O2 is being consumed in the
breakdown of organic matter but it's still pretty
good. It would have started near 100% around April
when the lake stratified
- The bubbles and wood chips Godfrey observed are
inconsistent with the O2 being so high in the
deeper waters. Methane production comes from the
sediment organic matter possibly including wood
chips from when the area last had a major cut
1. Such bubbling doesn't happen when
you have that much O2 remaining in the hypolimnion
unless the methane release is from deeper sediment
layers (ie there is still O2 in the near-surface
sediments to oxidize methane from deeper layers).
So that's good. If you run out of deep water O2,
the sediment gets stirred up by the methane:
nutrients and sediment are returned to the water
column
- So keep an eye on the O2 near the bottom
Notes: 1. Area has not been cut.
|